-
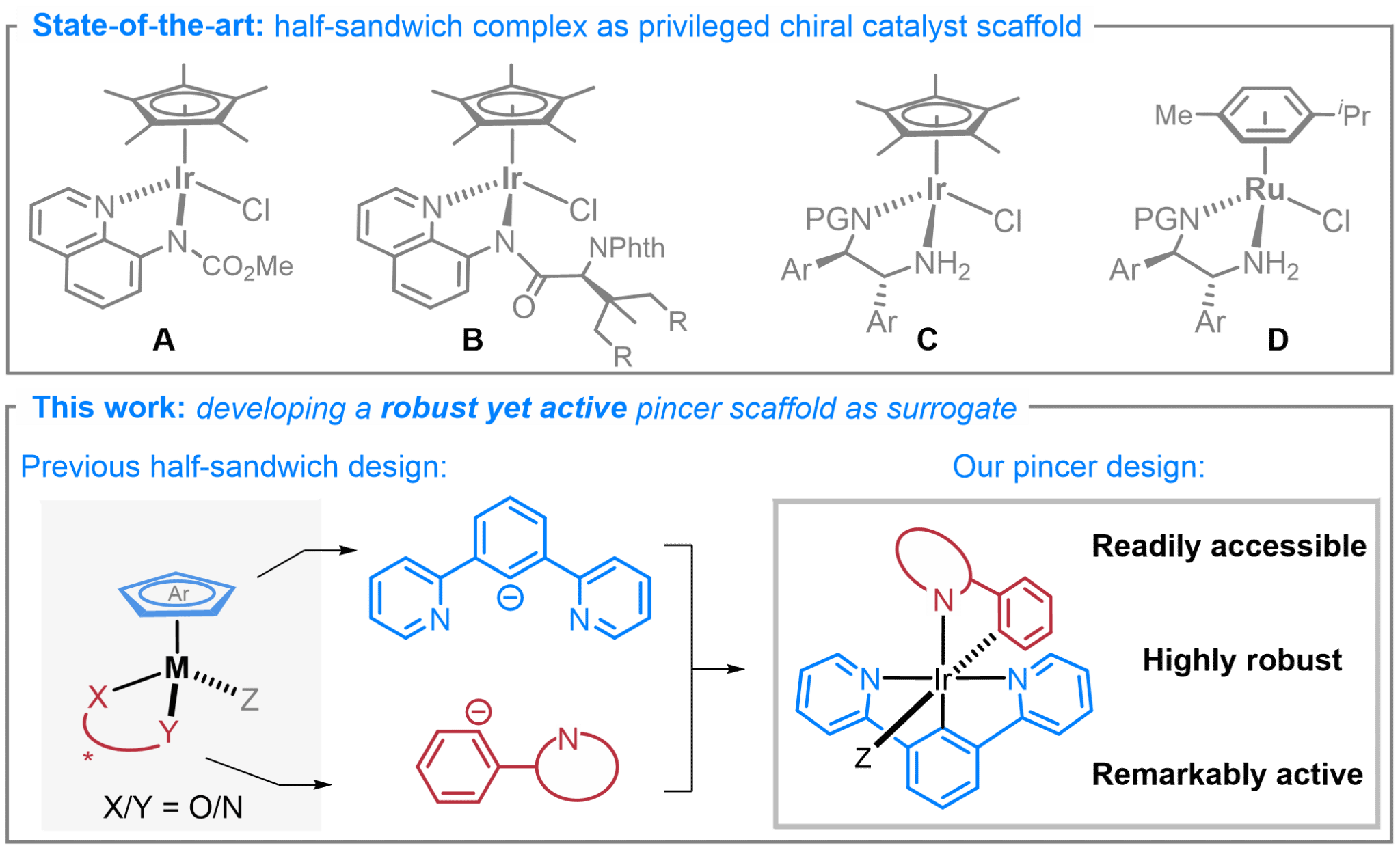
10. Ir(III) Pincer Complex with Solo Coordinatively Active Site as a Surrogate for Half-Sandwich Catalysts: Enabling Asymmetric Acylnitrenoid Transfer and Structure-Activity Relationship Studies. J. Am. Chem. Soc. 2025, 147, 38615.
Chu, Y.-P.†; Xu, Z.-H.†; Zhao, J.; Wang, C.; Daniliuc C. G.; Ma, J.* (†Equal contribution)
-
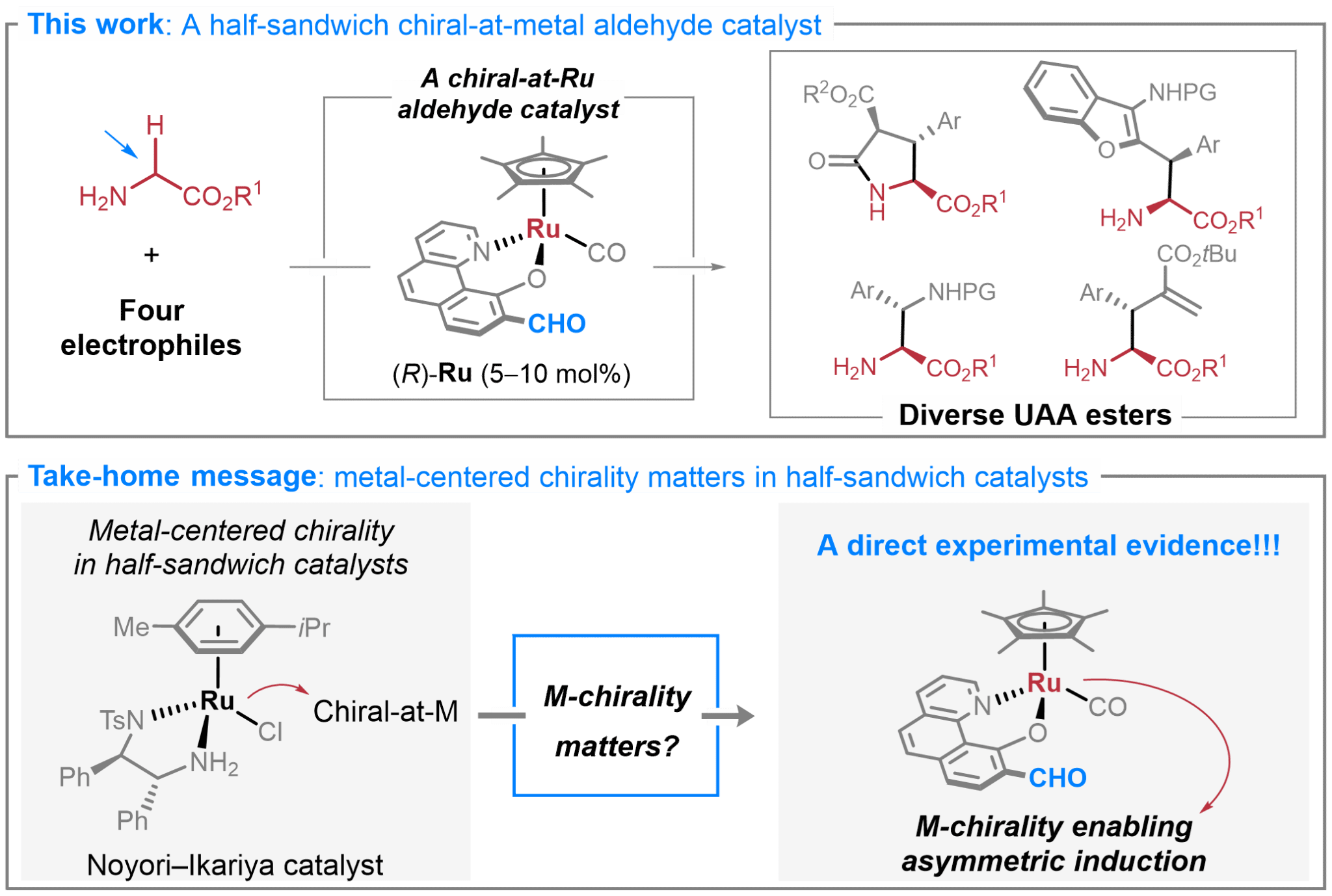
9. Accessing N-Unprotected Unnatural α-Amino Acid Esters by Half-Sandwich Chiral-at-Ruthenium Aldehyde Catalysis: Scope and Mechanistic Study. Angew. Chem. Int. Ed. 2025, e202510811.
Chen, G.†; Morais, G. N.†; Liang H.; Zhao, J.; Wang, C.; Chen, S.*; Ma, J.* (†Equal contribution)
A related preprint version was posted in ChemRxiv. 2025, doi:10.26434/chemrxiv-2025-xsk7p.
Highlighted in Synfacts (Synfacts 2025, 1266)
-
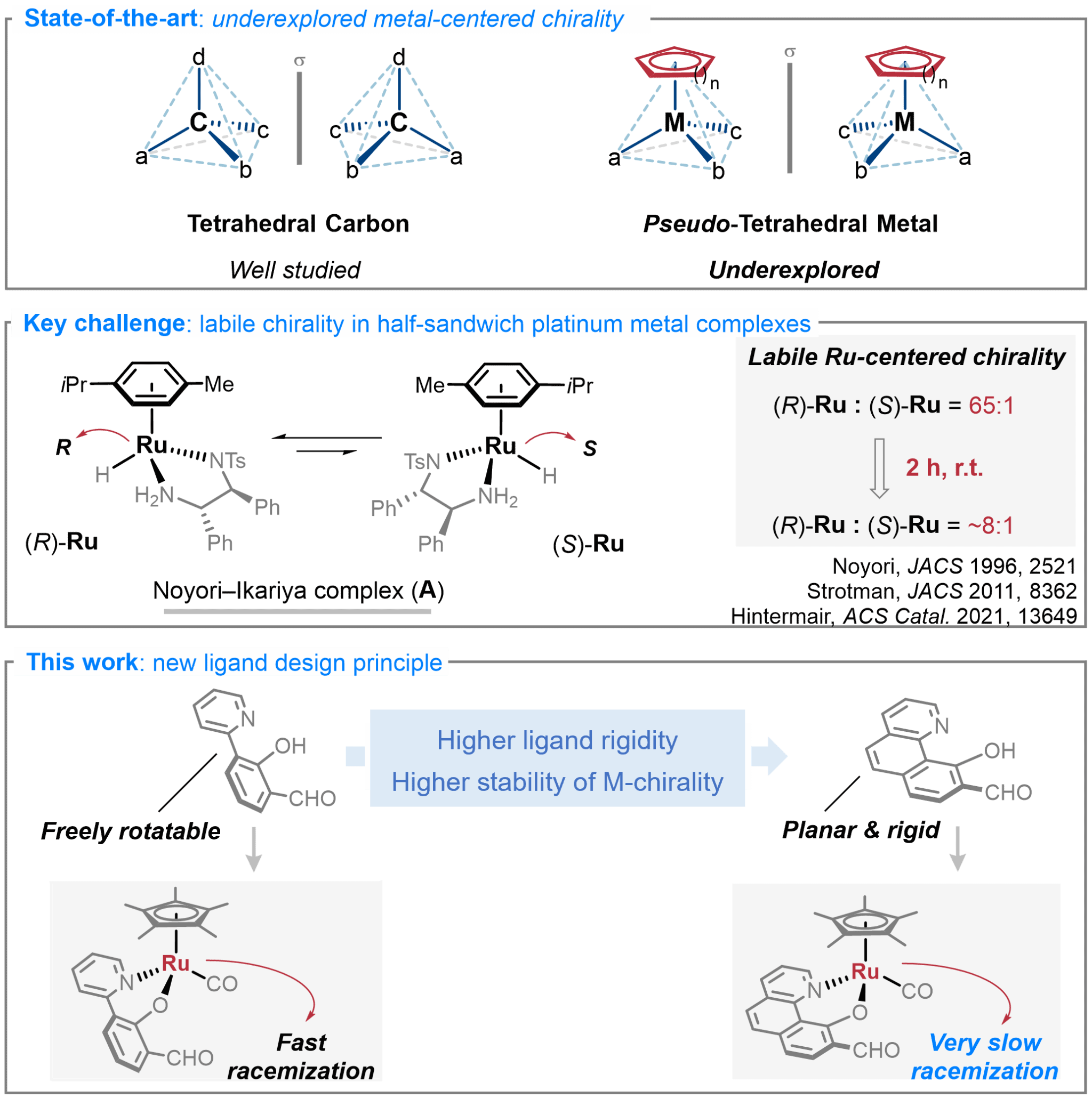
8. Half-Sandwich Ru(II) Complexes Featuring Metal-Centered Chirality: Configurational Stabilization by Ligand Design, Preparation via Kinetic Resolution, and Application in Asymmetric Catalysis. J. Am. Chem. Soc. 2025, 147, 6825.
Liang, H.†; Morais, G. N.†; Chen, G.†; Tang, W.; Zhao, J.; Wang, C.; Houk, K. N.; Chen, S.*; Ma, J.* (†Equal contribution)
-
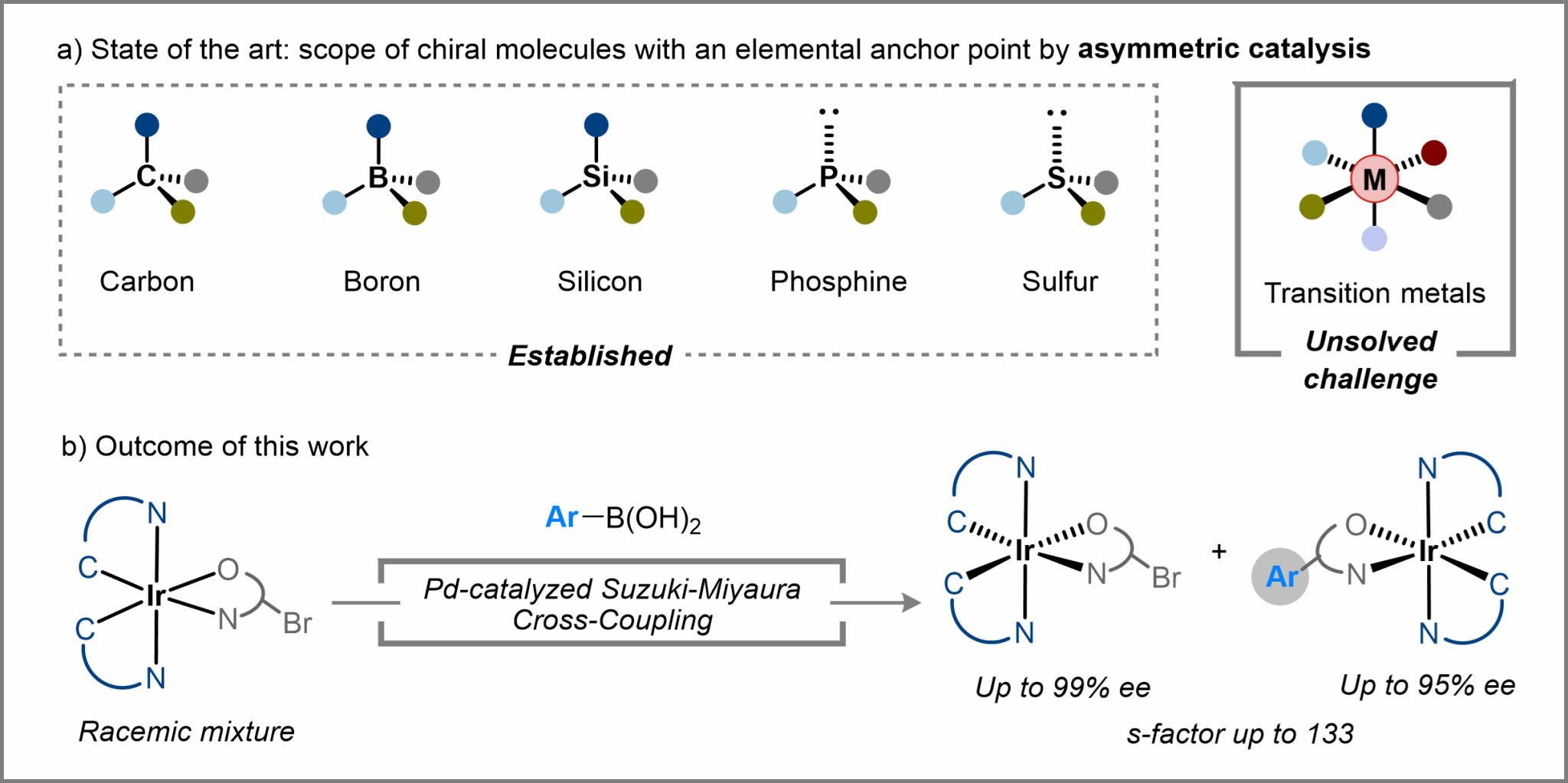
7. Asymmetric Synthesis of Stereogenic-at-Iridium(III) Complexes through Pd-Catalyzed Kinetic Resolution. Nat. Commun. 2025, 16, 1177.
Chu, Y.-P.; Yue, X.-L.; Liu, D.-H.; Wang C.; Ma, J.*
A related preprint version was posted in ChemRxiv. 2024, doi:10.26434/chemrxiv-2024-krgpl-v2.
-
6. Reshaping Quinolines by Single-Electron-Transfer-Triggered Dearomatization. Chem 2024, 10, 3529.
Chu, Y.-P.; Ma, J.*
-
5. Programmable Modular Assembly of Homochiral Ir(III)-Metallohelices to Reverse Metallodrug Resistance by Inhibiting CDK1. Angew. Chem. Int. Ed. 2024, 64, e202419292.
Li, X.*; Zhao, X.; Wang, X.; Xiong, A.; Wang, Z.; Shi, Z.; Zhang, J.; Wang, H.; Wei, W.; He, C.; Ma, J.; Guo, Z.; Duan, C.*; Zhao, J.*; Wang, X.*
-
4. 八面体金属中心手性络合物的合成研究进展. 中国基础科学, 2024, 26. DOI:10.3969/j.issn.1009-2412.2024.05.005. (Invited review, in Chinese)
楚云鹏, 马佳佳*
-
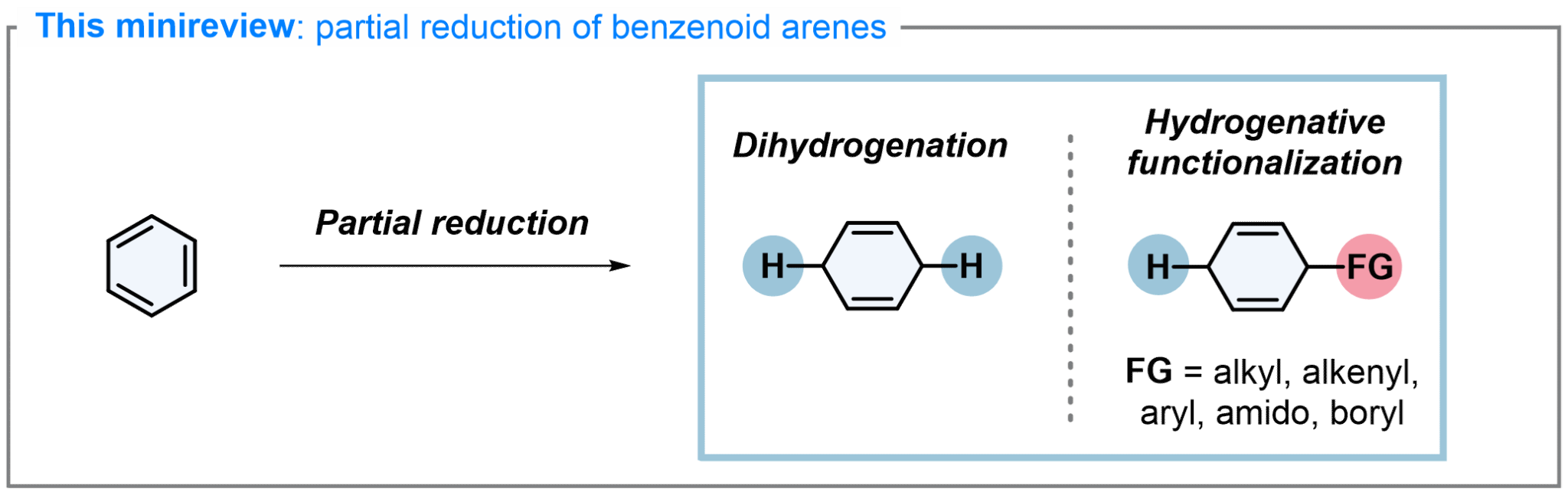
3. Recent Advances in Dearomative Partial Reduction of Benzenoid Arenes. Angew. Chem. Int. Ed. 2024, 63, e202402819.
Liu, D.-H.; Ma, J.*
-
2. Late-Stage Saturation of Drug Molecules. J. Am. Chem. Soc. 2024, 146, 11866.
Liu, D.-H.;† Pflüger P. M.;† Outlaw A.;† Lückemeier L.; Zhang F.; Regan C.; Nodeh H. R.; Cernak T.*; Ma, J.*; Glorius F.* (†Equal contribution)
Highlighted by Roche and Pfizer chemists Stepan & Richardson in Synfacts (Synfacts 2024, 0752)
Highlighted in Chemistry World NEWS (Late-stage saturation could improve drugs' effectiveness)
Top 20 Most Read JACS Articles in 2024
-
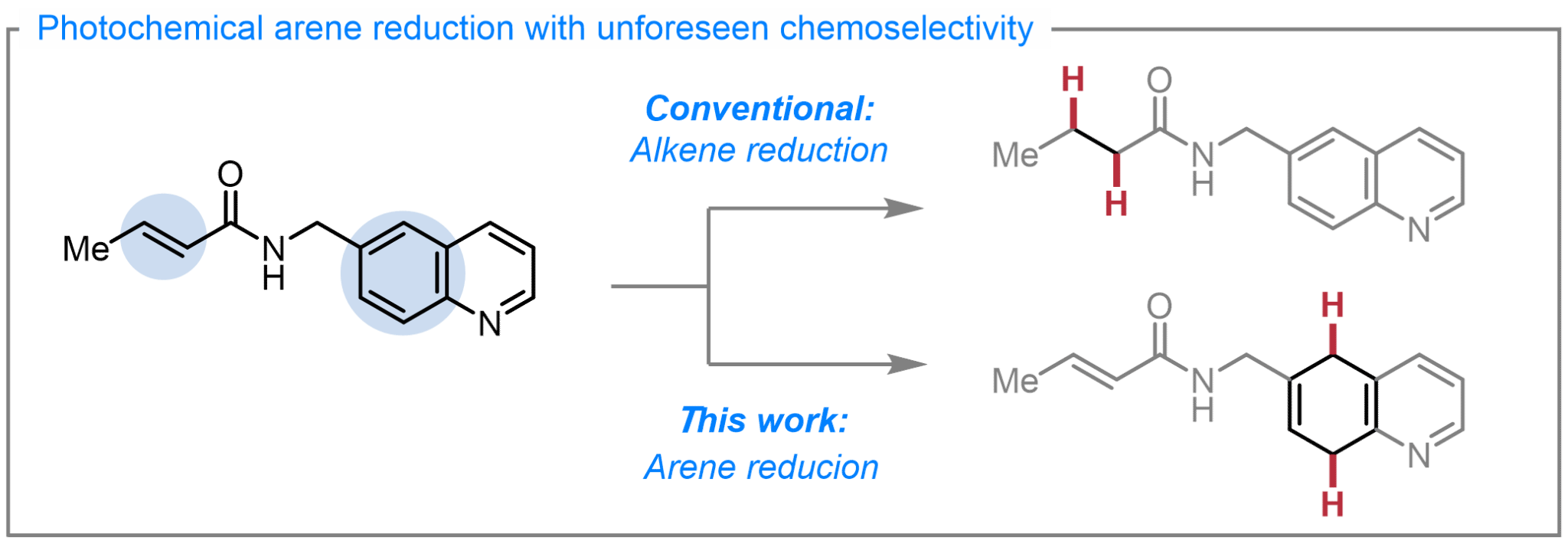
1. Chemoselective Quinoline and Isoquinoline Reduction by Energy Transfer Catalysis Enabled Hydrogen Atom Transfer. Angew. Chem. Int. Ed. 2023, 62, e202312203.
Liu, D.-H.;† Nagashima, K.;† Liang, H.; Yue, X.-L.; Chu, Y.-P.; Chen, S.*; Ma, J.* (†Equal contribution)
Highlighted in Organic Chemistry Portal
Top 10% Most Viewed Angew Chemie Papers Published in 2023
Applied as key step in total synthesis of annotinolide by M. W. Smith's group
-
Prior to SJTU
-
15. Ma, J.;† Chen, S.;† Bellotti, P.;† Wagener, T.; Daniliuc, C.; Houk, K. N.; Glorius, F. Facile access to fused 2D/3D rings via intermolecular cascade dearomative [2+2] cycloaddition/rearrangements of quinolines with alkenes. Nat. Catal. 2022, 5, 405. (†Equal contribution)
-
14. Ma, J.;† Chen, S.;† Bellotti, P.;† Guo, R.; Schäfer, F.; Heusler, A., Zhang, X., Daniliuc, C., Brown, M. K.; Houk, K. N.; Glorius, F. Photochemical intermolecular dearomative cycloaddition of bicyclic azaarenes with alkenes. Science 2021, 371, 1338. (†Equal contribution)
-
13. Huang, H.-M.; Bellotti, P.; Ma, J.; Dalton, T.; Glorius, F. Bifunctional reagents in organic synthesis. Nat. Rev. Chem. 2021, 5, 301.
-
12. Ma, J.; Schäfers, F.; Daniliuc, C.; Bergander, K.; Strassert, C. A.; Glorius, F. Gadolinium photocatalysis: Dearomative [2+2] cycloaddition/ring-expansion sequence with indoles. Angew. Chem. Int. Ed. 2020, 59, 9639.
-
11. Ma, J.; Strieth-Kalthoff, F.; Dalton, T.; Freitag, M.; Schwarz, J. L.; Bergander, K.; Daniliuc, C.; Glorius, F. Direct dearomatization of pyridines via an energy-transfer-catalyzed intramolecular [4+2] cycloaddition. Chem 2019, 5, 2854. (Cover art, 封面文章)
-
10. Patra, T.; Mukherjee, S.; Ma, J.; Strieth-Kalthoff, F.; Glorius, F. Visible-light-photosensitized aryl and alkyl decarboxylative functionalization reactions. Angew. Chem. Int. Ed. 2019, 58, 10514.
-
9. Ma, J.; Lin J.; Zhao L.; Harms K.; Marsch M.; Xie X.; Meggers, E. Synthesis of beta-substituted gama-aminobutyric acid derivatives via enantioselective photoredox catalysis. Angew. Chem. Int. Ed. 2018, 57, 11193.
-
8. Ma, J.;† Zhang, X.;† Huang, X.; Luo, S.; Meggers, E. Preparation of chiral-at-metal catalysts and their use in asymmetric photoredox chemistry. Nat. Protoc. 2018, 13, 605. (†Equal contribution)
-
7. Ma, J.; Xie, X.; Meggers, E. Catalytic asymmetric synthesis of fluoroalkyl‐containing compounds by three‐component photoredox chemistry. Chem. Eur. J. 2018, 24, 259.
-
6. Ma, J.; Rosales, R. A.; Huang, X., Harms, K.; Riedel, R.; Wiest, O.; Meggers, E. Visible-light-activated asymmetric beta-C–H functionalization of acceptor-substituted ketones with 1,2-dicarbonyl compounds. J. Am. Chem. Soc. 2017, 139, 17245.
-
5. Ma, J.; Harms, K.; Meggers, E. Enantioselective rhodium/ruthenium photoredox catalysis en route to chiral 1,2-aminoalcohols. Chem. Commun. 2016, 52, 10183.
-
4. Ma, J.; Shen, X.; Harms, K.; Meggers, E. Expanding the family of bis-cyclometalated chiral-at-metal rhodium(iii) catalysts with a benzothiazole derivative. Dalton Trans. 2016, 45, 8320.
-
3. Ma, J.; Ding, X.; Hu, Y.; Huang, Y.; Gong, L.; Meggers, E. Metal-templated chiral Brønsted base organocatalysis. Nat. Commun. 2014, 5, 4531.
-
2. Chen, L.-A.; Xu, W.; Huang, B.; Ma, J.; Wang, L.; Xi, J.; Harms, K.; Gong, L.; Meggers, E. Asymmetric catalysis with an inert chiral-at-metal iridium complex. J. Am. Chem. Soc. 2013, 135, 10598.
-
1. Chen, L.-A.; Ma, J.; Celik, M. A.; Yu, H.-L.; Cao, Z.; Frenking, G.; Gong, L.; Meggers, E. Active versus passive substituent participation in the auxiliary-mediated asymmetric synthesis of an octahedral metal complex. Chem. Asian J. 2012, 7, 2523.


