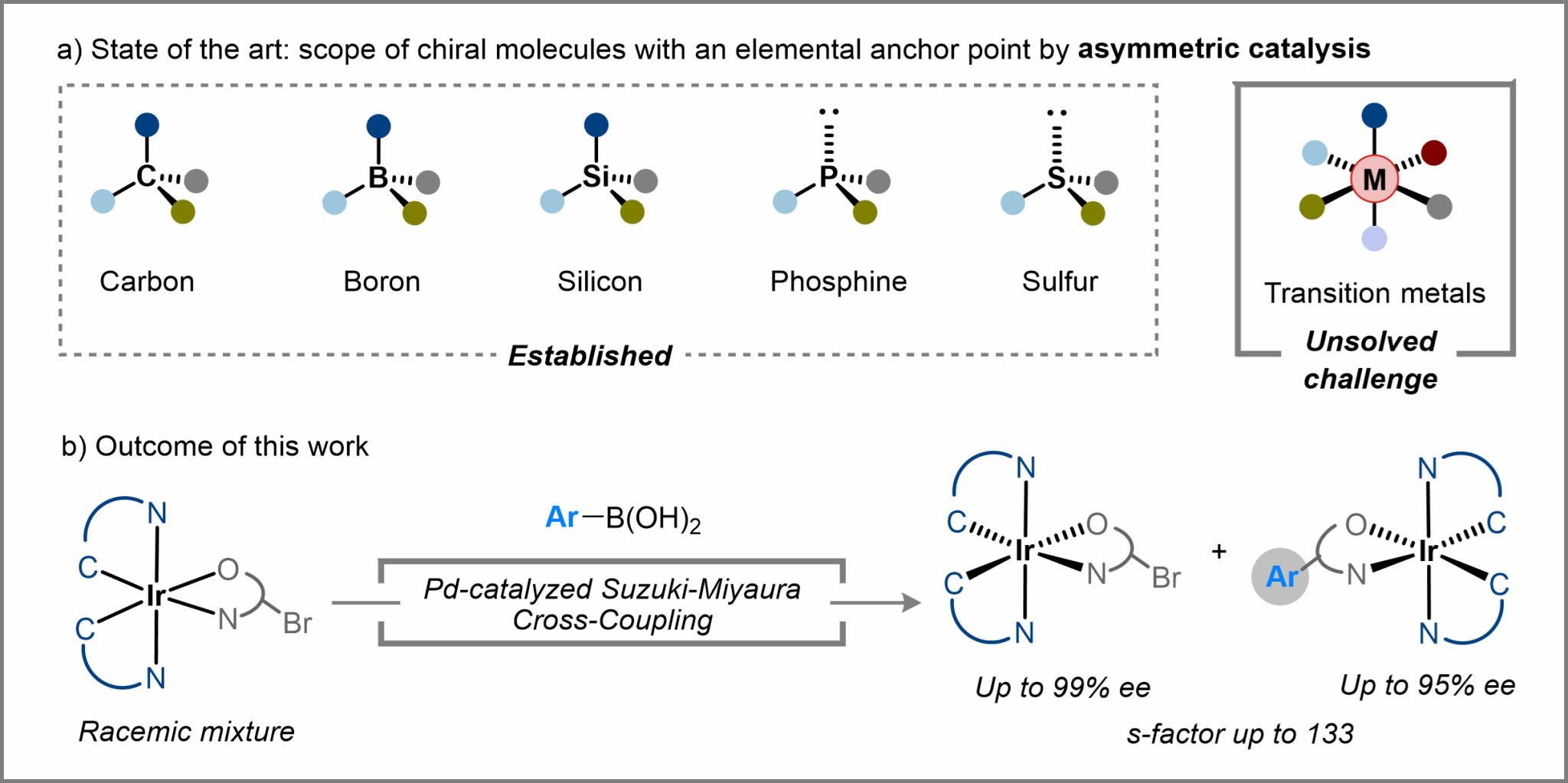
Metal-centered chirality has been recognized for over one century. However, synthesis of stereogenic-at-metal complexes remains constrained.
Notably, while asymmetric catalysis has played a crucial role in the production of optically active organic molecules, its application to stereogenic-at-metal complexes is less straightforward.
In this study, we introduce a kinetic resolution strategy employing a Pd-catalyzed asymmetric Suzuki-Miyaura cross-coupling reaction that efficiently produces optically active stereogenic-at-iridium complexes from racemic mixtures with high selectivity (achieving an s-factor of up to 133). This method enables further synthesis of complexes relevant to chiral metallodrugs and photosensitizers, underscoring the practical utility of our approach. Mechanistic studies suggest that reductive elimination is likely the turnover-limiting step over the Suzuki-Miyaura cross-coupling.
Please kindly check our latest publication in Nature Communications:https://www.nature.com/articles/s41467-024-55341-4.pdf
Congratulations to Yun-Peng and the entire team on this achievement!